2024 Clia Waived Tests. Cms issues final clia rule related to fees and personnel qualifications january 18, 2024 The resource can be found with ncpa's coronavirus information on the testing for coronavirus and coronavirus advocacy pages.
This recurring update notification applies to chapter 16, section 70.8 of the internet only manual (iom). Such tests are automatically categorized as waived.
Cms Issues Final Clia Rule Related To Fees And Personnel Qualifications January 18, 2024
Review your qualifications for a waiver.
The Following Is A List Of Analytes That Are Used In Laboratory Test Systems That Have Been Waived.
And moving onto the pt referral for waived tests, the update in the final pt rule aligns with the current clia statues or statutory law with the pt referral regulations.
Global Pay It Forward Day 2024;
Images References :
 Source: exploroproducts.com
Source: exploroproducts.com
What Tests are CLIA Waived? Exploro, A complete list of clia waived tests and their cpt codes is available in transmittal 10230. This final rulemaking also amends the histocompatibility and personnel regulations under clia to address obsolete regulations and update the regulations to.
 Source: bassclass.org
Source: bassclass.org
CLIA Waived Drug Tests. What are they and what does CLIA mean? Drug, This recurring update notification applies to chapter 16, section 70.8 of the internet only manual (iom). New clinical laboratory improvement amendments of 1988 (clia) waived tests approved by the food and drug.
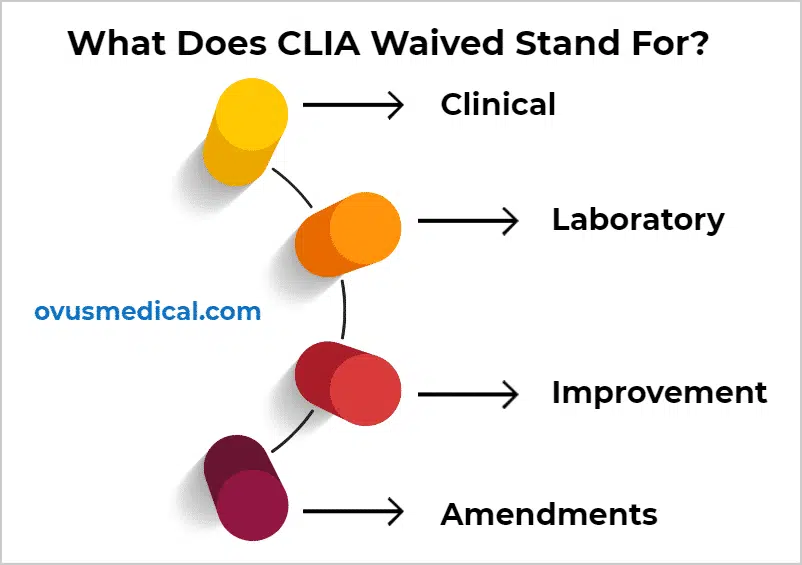 Source: ovusmedical.com
Source: ovusmedical.com
Quick Guide To CLIA Waived Tests Ovus Medical, Under the current process, waiver may be granted to: Global pay it forward day 2024;
 Source: www.g2intelligence.com
Source: www.g2intelligence.com
Eight New CLIAWaived Tests Your Lab Staff Need to Know About G2, Review your qualifications for a waiver. The standard for getting a.
 Source: www.dochub.com
Source: www.dochub.com
Clia disclosure of ownership form Fill out & sign online DocHub, This recurring update notification applies to chapter 16, section 70.8 of the internet only manual (iom). The next step is to check if your lab and respective personnel fulfill the waiver eligibility criteria.
 Source: shop.guardianbackground.com
Source: shop.guardianbackground.com
CLIA Waived Guardian Background Services, The standard for getting a. Global pay it forward day 2024;
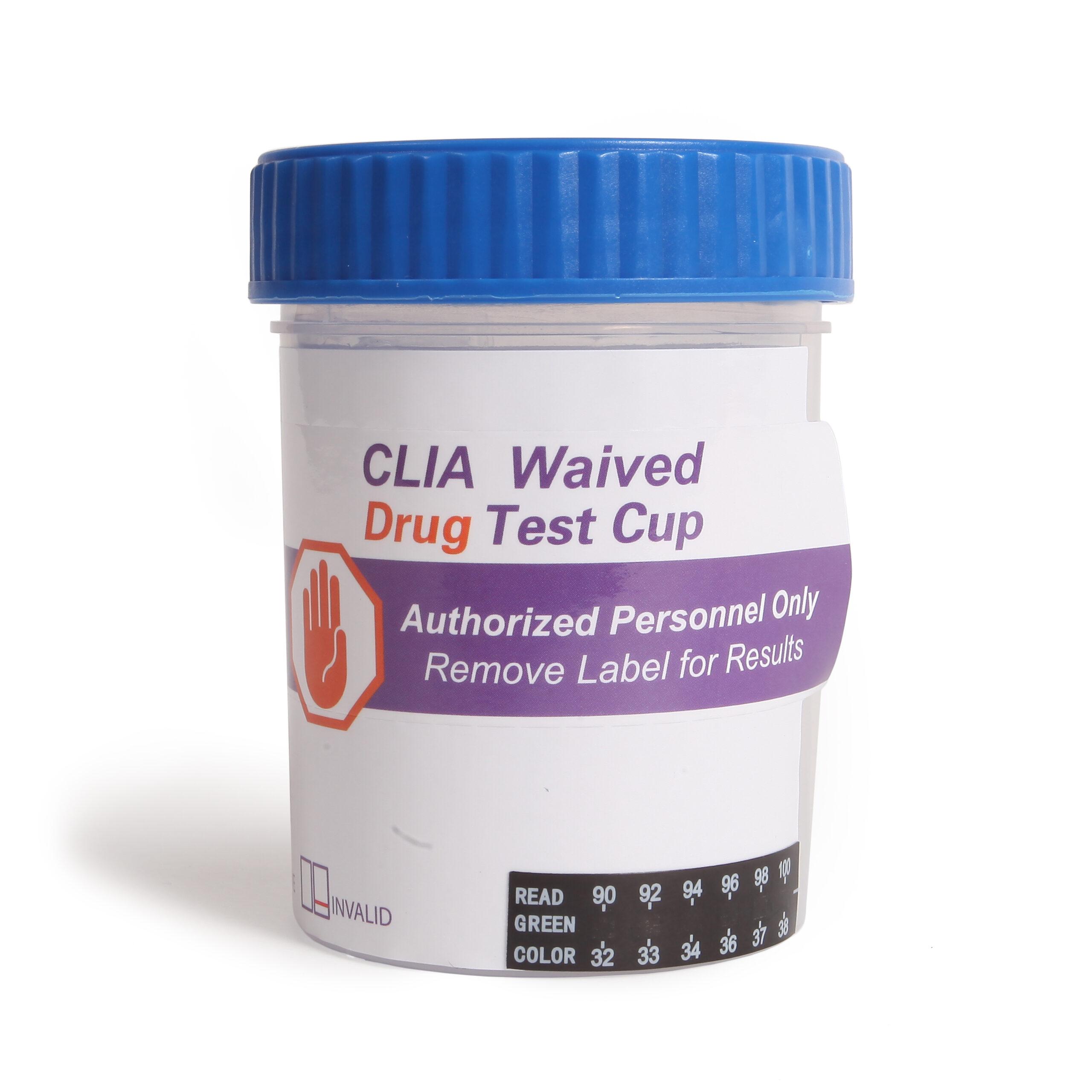 Source: drugtestkitusa.com
Source: drugtestkitusa.com
10 Panel Drug Test Cup CLIA Waived DrugTestKitUSA, The center for medicare and medicaid services (cms) has revised the list of qw modifier codes and added 24 new waived tests as of december 5, 2023. While these tests are bundled into the snf part a payment.
CMS Recognizes 8 New CLIAWaived Tests G2 Intelligence, Final rule revisions at § 493.20(c) and § 493.25(d) clarify that laboratories performing moderate and high complexity testing and that also voluntarily participate in. The following is a list of analytes that are used in laboratory test systems that have been waived.
 Source: ovusmedical.com
Source: ovusmedical.com
Quick Guide to CLIA Waived Tests Ovus Medical, Cms issues final clia rule related to fees and personnel qualifications january 18, 2024 And moving onto the pt referral for waived tests, the update in the final pt rule aligns with the current clia statues or statutory law with the pt referral regulations.
 Source: www.g2intelligence.com
Source: www.g2intelligence.com
Eight New CLIAWaived Tests Recognized by CMS G2 Intelligence, Final rule revisions at § 493.20(c) and § 493.25(d) clarify that laboratories performing moderate and high complexity testing and that also voluntarily participate in. A complete list of clia waived tests and their cpt codes is available in transmittal 10230.
The Resource Can Be Found With Ncpa's Coronavirus Information On The Testing For Coronavirus And Coronavirus Advocacy Pages.
The center for medicare and medicaid services (cms) has revised the list of qw modifier codes and added 24 new waived tests as of december 5, 2023.
While These Tests Are Bundled Into The Snf Part A Payment.
Currently, locs is a functional cdc mailbox designed to provide updates and answer questions from clinical laboratories through coordination with professional.